Chronic Infection
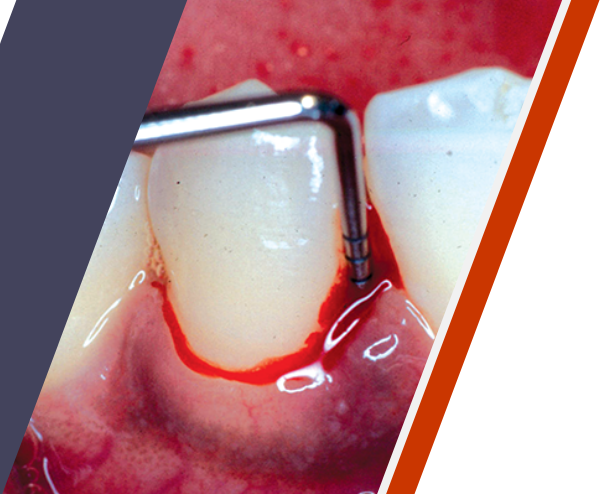
Periodontitis can be a serious chronic infection3
Periodontitis, also known as gum disease, may be associated with an increased risk for other chronic conditions throughout the body such as cardiovascular disease.4,5
Also, certain chronic conditions are risk factors for periodontitis.6
Keystone
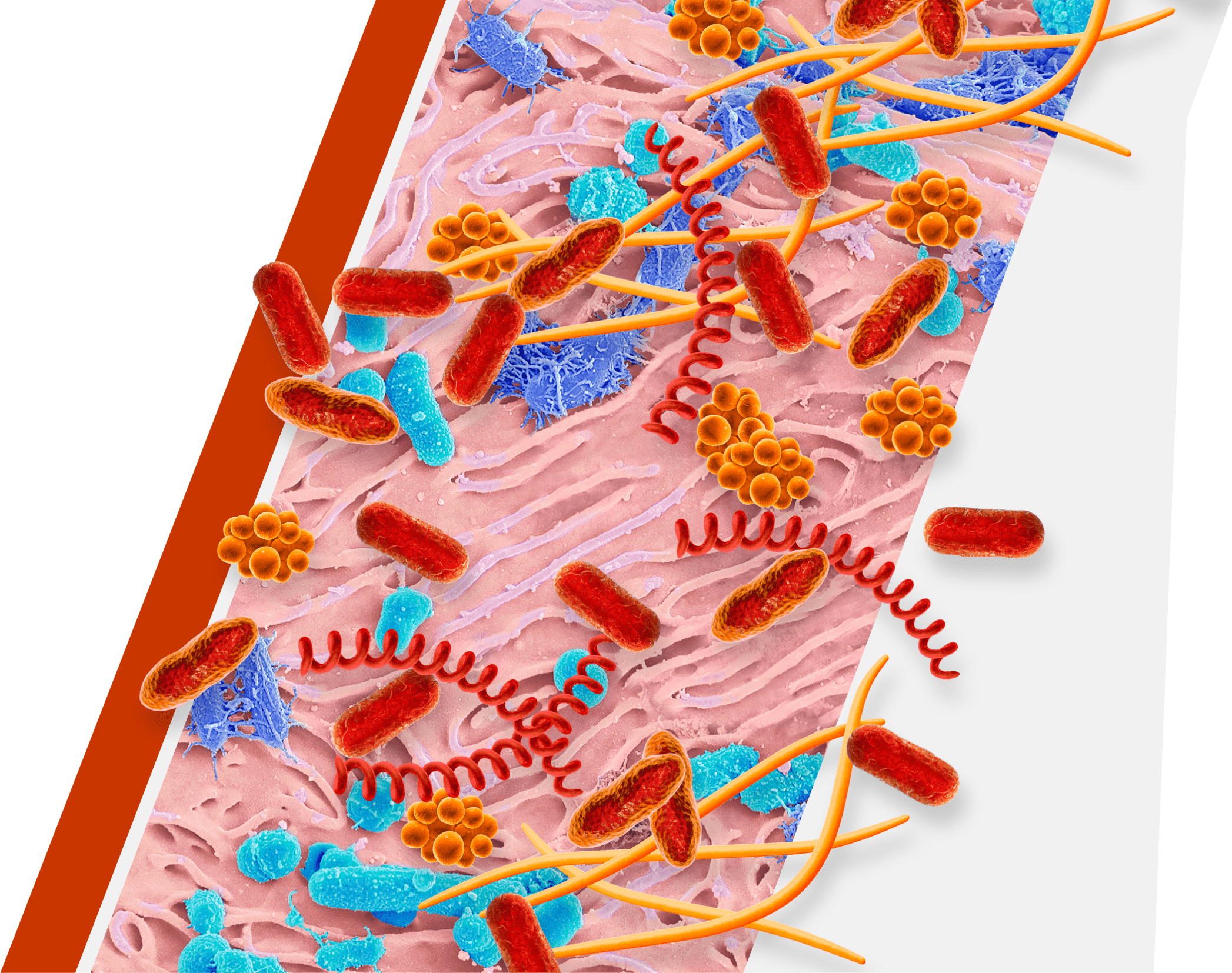
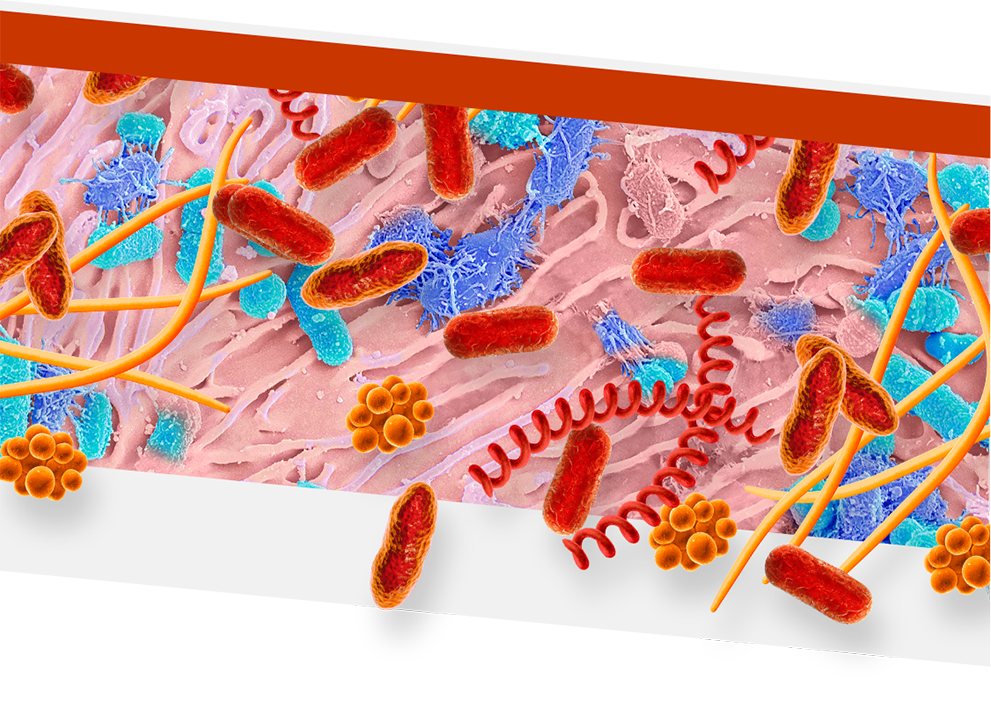
An overgrowth of keystone pathogens, including red and orange complex bacteria, within the oral microbiome can lead to periodontitis7-12
- Keystone pathogens may be associated with other diseases13,14
Keystone


Keystone pathogens tell a colorful story
Orange Complex Bacteria
- F nucleatum
- P micra

Red Complex Bacteria
- P gingivalis
- T denticola
- T forsythia

- Orange complex bacteria are thought to bridge earlier bacteria that appear on the tooth surface with red complex bacteria15,16
- As periodontitis progresses, the dominance of orange complex bacteria shifts in favor of red complex bacteria9
- Red complex bacteria are the most common and destructive keystone pathogens10,11
- P gingivalis can enter and exit gingival cells to allow for persistent infection17
Learn about the limitations of SRP
REFERENCES: 1. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018;149(7):576-588. doi:10.1016/j.adaj.2018.04.023 2. Periodontal disease in adults (age 30 or older). National Institute of Dental and Craniofacial Research. Updated August 2021. Accessed July 5, 2023. https://www.nidcr.nih.gov/research/data-statistics/periodontal-disease/adults 3. Sudhakaran S, Ali S, Reshma TS, Rajendran P, Sayd S. Personalized periodontal treatment: a new paradigm shift. EC Dent Sci. 2020;(19.8):136-141. 4. Borojevic T. Smoking and periodontal disease. Mater Sociomed. 2012;24(4):274-276. doi:10.5455/msm.2012.24.274-276 5. Sanz M, del Castillo AM, Jepsen S, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47(3):268-288. doi:10.1111/jcpe.13189 6. Polak D, Sanui T, Nishimura F, Shapira L. Diabetes as a risk factor for periodontal disease–plausible mechanisms. Periodontol 2000. 2020;83(1):46-58. doi:10.1111/prd.12298 7. Lenartova M, Tesinska B, Janatova T, et al. The oral microbiome in periodontal health. Front Cell Infect Microbiol. 2021;11:629723. doi:10.3389/fcimb.2021.629723 8. Radiac A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Comp Struc Biotech J. 2021;19:1335-1360. doi:10.1016/j.csbj.2021.02.010 9. Mohanty R, Asopa SJ, Joseph MD, et al. Red complex: polymicrobial conglomerate in oral flora: a review. J Fam Med Prim Care. 2019;8:3480-3486. 10. Hajishengallis G, Darveau RP, Curtis MA. The keystone pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717-725. doi:10.1038/nrmicro2873 11. Sedghi LM, Bacino M, Kapila YL. Periodontal disease: the good, the bad, and the unknown. Front Cell Infect Microbiol. 2021;11:1026. doi:10.3389/fcimb.2021/766944 12. Socransky SS, Hajjafee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135-187. 13. Colombo APV, Magalhães CB, Hartenbach FARR, do Souto RM, de Silva-Boghossian CM. Periodontal-disease-associated biofilm: a reservoir for pathogens of medical importance. Microb Pathog. 2016;94:27-34. doi:10.1016/jmicpath.2015.09.009 14. Peng X, Cheng L, Tang C, et al. Oral microbiota in human systemic diseases. Int J Oral Sci. 2022;14:14. doi:10.1038/s41368-022-00163-7 15. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12-55. doi:10.1034/j.1600-0757.2002.280102.x 16. Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134-144. doi:10.1111/j.1600-051x.1998.tb02419.x 17. Sakanaka A, Takeuchi H, Kuboniwa M, Amano A. Dual lifestyle of Porphyromonas gingivalis in biofilm and gingival cells. Microb Pathog. 2016;94:42-47. doi:10.1016/j.micpath.2015.10.003