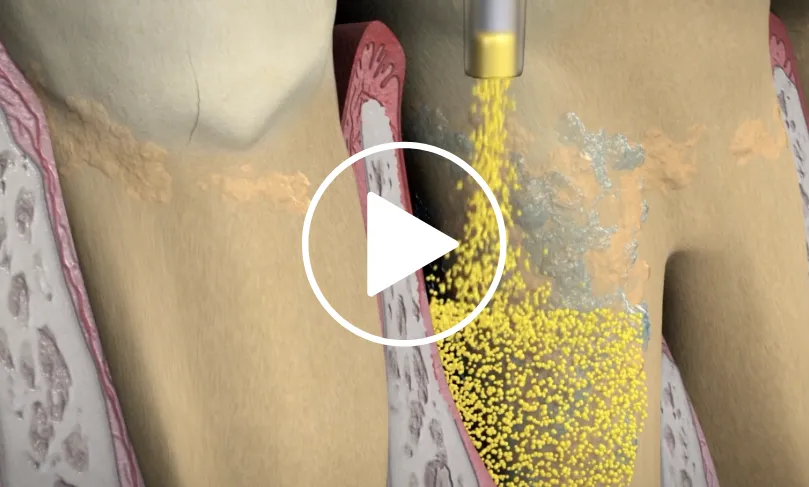
Administration
ARESTIN is provided as a dry powder, packaged in a unit-dose cartridge with a deformable tip, which is inserted into a spring-loaded cartridge handle mechanism.1

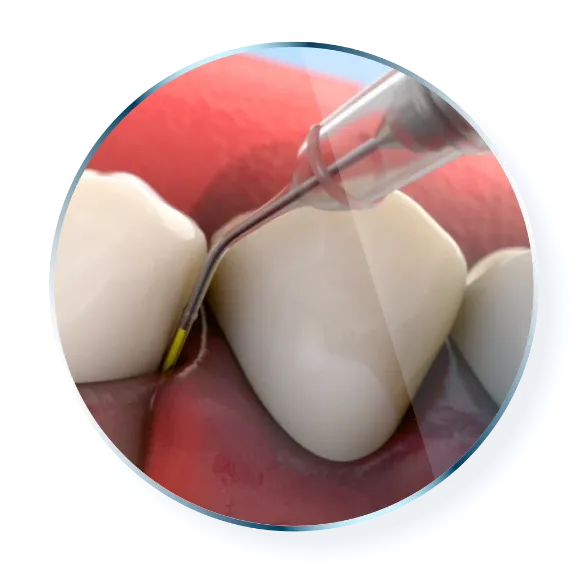
Tips for Use
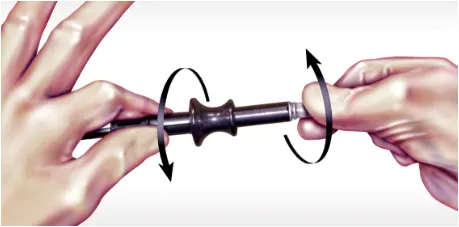
Insert the cartridge into the handle while exerting slight pressure. Twist until you align the notch on the cartridge with the groove on the handle and you hear the cartridge “lock” into place. Remove the cap on the tip.
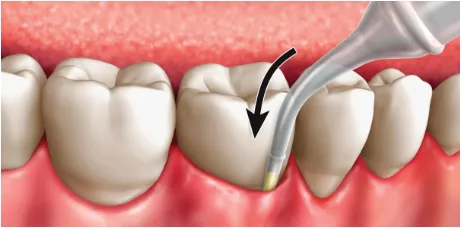
Place the cartridge tip into the periodontal pocket, parallel to the long axis of the tooth. Be sure not to force the tip into the base of the pocket.
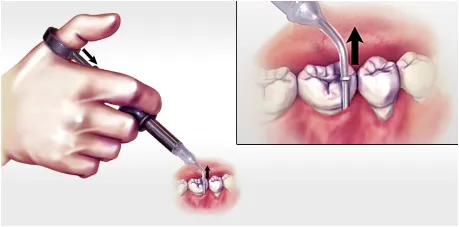
Gently press the thumb ring to express the ARESTIN powder while withdrawing the cartridge tip away from the base of the pocket. If you feel any resistance during delivery, further withdraw the device.
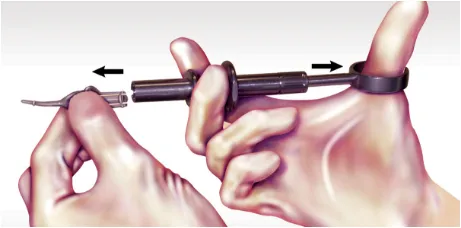
Once delivery is complete, retract the thumb ring and remove the ARESTIN cartridge with your free hand. Appropriately discard the cartridge and sterilize the handle prior to reuse.
Professional subgingival administration is accomplished by inserting the unit-dose cartridge to the base of the periodontal pocket and then pressing the thumb ring in the handle mechanism to expel the powder while gradually withdrawing the tip from the base of the pocket.1
No local anesthesia required1
No adhesive or dressing required1
No need to remove ARESTIN, as it is bioresorbable1
The handle mechanism should be sterilized between patients1
REFERENCE: 1. ARESTIN® (minocycline hydrochloride) microspheres, 1 mg. Prescribing Information. OraPharma; Bridgewater, NJ.
IMPORTANT SAFETY INFORMATION AND INDICATION
- ARESTIN® (minocycline HCl) is contraindicated in any patient who has a known sensitivity to minocycline or tetracyclines. Hypersensitivity reactions and hypersensitivity syndrome that included, but were not limited to anaphylaxis, anaphylactoid reaction, angioneurotic edema, urticaria, rash, eosinophilia, and one or more of the following: hepatitis, pneumonitis, nephritis, myocarditis, and pericarditis may be present. Swelling of the face, pruritus, fever and lymphadenopathy have been reported with the use of ARESTIN. Some of these reactions were serious. Post-marketing cases of anaphylaxis and serious skin reactions such as Stevens Johnson syndrome and erythema multiforme have been reported with oral minocycline, as well as acute photosensitivity reactions.